Microbiology
 The Comparative Pathology Laboratory is able to offer decades of combined experience performing aerobic bacterial cultures of mice, rats, and other veterinary research species such as zebrafish and additional aquatic species. We routinely perform:
The Comparative Pathology Laboratory is able to offer decades of combined experience performing aerobic bacterial cultures of mice, rats, and other veterinary research species such as zebrafish and additional aquatic species. We routinely perform:
- Routine health monitoring cultures.
- Cultures of clinically significant tissues/lesions.
- Environmental monitoring cultures, including water cultures.
- Autoclave testing.
Please contact us for questions regarding sample submission.
Test |
Description |
Rate Code |
|---|---|---|
| Aerobic culture | Culture for isolation and identification of aerobic bacteria or fungi from a single site or sample | M010 |
| Anaerobic culture | Culture for isolation and identification of anaerobic bacteria | 0055 |
| Special stains | Gram with interpretation for identification of microorganisms | M040 |
| Autoclave spore ampule testing | Includes spore ampules with testing, interpretation and controls | M050 |
| Rodac plate testing | Includes incubation and grid counting with report generation for each site tested (up to 10 plates). Plates are included in the fee, but media must be picked up prior to submission. Special stains: we do not do Acid fast stains, only Gram stains. | M060 |
| Water sample testing | Water sample filtration for particulates with aerobic culture and pH | M070 |
| Environmental swab testing | Culture for isolation and identification of aerobic bacteria or fungi from an environmental swab | M080 |
Microbiology Submission Guidelines
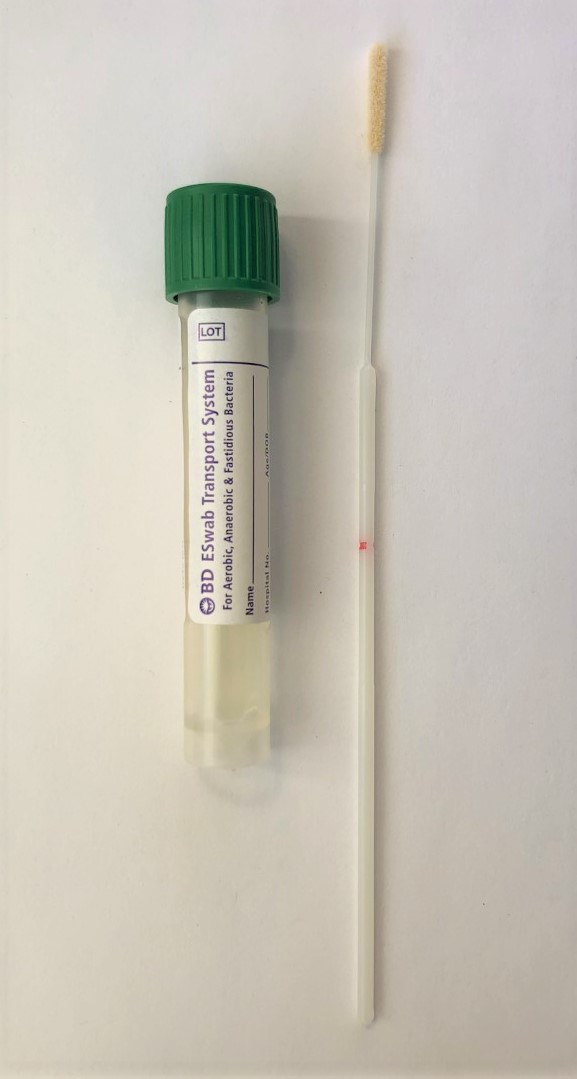
Using the BD Eswab Minitip Collection Kit (Becton Dickinson, Catalog # 220246), aseptically swab the lesion of interest using the flocked end of the wand. After collection, snap the flocked end of the wand into the elution tube at the red line. Label the tube with the animal ID, tissue cultured, and date. Place the tube in a secondary container, such as a zipper storage bag, and ship overnight on ice packs.
